Without combating the varroa mite, a bee colony will die within one to three years [1]. Honeybees are therefore dependent on varroa control. But which treatment is the best? We have summarized the advantages and disadvantages of the current treatment methods for you and give an insight into the current research on this topic.
Chemical Method
The chemical treatment is based on an increased toxicity of certain substances to varroa mites compared to honeybees. However, since both species belong to the arthropods, they are similar in metabolism. This means that substances used on varroa mites are poisonous also for the honey bee. However, the effect is smaller due to the larger body mass (higher LD50) of the honey bee[2]. In general, there are two forms of chemicals: synthetic and natural chemicals.
Synthetic chemicals
The only synthetic substance approved in Switzerland for varroa control is Coumaphos. However, the first resistant varroa mites were discovered in northern Italy in 1999, so it will only be a matter of time before the treatment becomes ineffective[3]. Additionally, Coumaphos is stored in beeswax which contaminates the wax cycle, over time the substance can reach harmful doses for bees[4]. At a particular level of contamination in the wax, smaller queens will develop. These queens have an increased probability to be insufficiently mated during her mating flights and because of it, will be unable to lay eggs[5-6]. At even higher levels of Coumaphos, no queens can develop at all [6]. We therefore strongly advise against the usage of Coumaphos for varroa treatment.
Natural chemicals
Although it is certainly better to rely on natural substances instead of synthetic ones, they can also be harmful to bees. In Switzerland, thymol and formic acid are mainly used for summer treatment and oxalic acid for winter treatment. All three chemicals trigger a stress reaction in the bees’ metabolism[7]. Oxalic acid has also been shown to accumulate in the digestive system of bees, causing organ damage[8]. All three substances increase bee mortality and slow down the colony’s development[9-15]. Both organic acids (formic and oxalic acid) can also cause queen losses [9,13].
Already in 2004 a reduced effectiveness of thymol was measured in Italy compared to earlier years[16], in Switzerland no further reduction of the varroa infestation could be observed this year after thymol treatments[17]. Therefore, formic acid can be considered as the more efficient alternative to summer treatment. However, the toxic dose for the honey bee is close to that of the varroa mite and is strongly temperature-dependent [2]. This makes it extremely difficult to apply the treatment effectively, without harming the bees, in an environment with fluctuating temperatures. Moreover, both summer treatments (formic acid and thymol) can contaminate the honey [18] and must therefore be applied after the honey harvest – a time when the varroa population has already risen considerably and is harming the bees.
Hyperthermy or thermal treatment
Hyperthermy or thermal treatment, is based on the honeybee’s greater heat tolerance compared to the varroa mite. If heat treatment is carried out at 42°C for a few hours, varroa mites will die whereas the bees will survive[19]. Hyperthermia follows a biological approach and can be used throughout the breeding season, regardless of honey harvest and weather. While the first attempts of conventional heat treatment did not lead to the hoped-for success, more recent approaches are very promising.
External heat treatment
The first heat treatments applied the heat from the outside and heated the entire hive. In response, the worker bees tried to cool the brood, making it difficult to control the temperature in the hive and exposing the workers to unnecessary stress. Such procedures repeatedly led to bee and queen losses and had only a limited effect against the varroa mite[20]. In order to avoid the cooling behaviour of the workers, all brood combs had to be removed from the colony and heat-treated separately. However, this method is very time-consuming for the beekeeper and was therefore unsuccessful.
Internal heat treatment
New heat treatment processes, which generate heat through a heating system built into the wax, are currently being developed. In this way, the Varroa mites are fought where their infestation is the strongest – namely, in the capped brood. At the same time the bees can keep the hive at the usual temperature and are not exposed to stress. Vatorex is currently testing such a system in cooperation with the Swiss Centre for Bee Research. Initial results have confirmed the effectiveness of this method, which is currently being optimized in terms of effectiveness and bee tolerance.
Biomechanical control
The Varroa mites reproduce inside the capped brood cells and show a strong preference for drones over worker brood. The two biomechanical treatment methods, aim to use this as an advantage.
Drone brood removal
By drone brood removal, empty frames are placed in the colony in spring, which the bees will use as brood comb. Subsequently, the drone comb is cut out one to three times – each time when the brood is mostly capped. Because the Varroa mites parasitize drone brood approx. 12 times more frequently than worker brood, this procedure can reduce Varroa infestation in spring by up to 80%[21].
However, the repeated removal of drones is a major intervention in the population dynamics of the honey bee. Although the drones are quite lazy and therefore of little use to us beekeepers, they still carry the genetic material of the next generations of young queens and workers. Drones thereby play an important role in maintaining genetic diversity, which is why we advise against removing drones more than once a year.
Comb trapping method
In the comb trapping method, the queen is sealed off from a particular brood comb, which is removed and melted down as soon as the brood is covered. By removing the comb three times in succession, 80% of the mites can be removed[22].
This method is recommended by increased mite infestation in spring and early summer, as chemical treatment at this time will cause honey contamination. Additionally, the procedure can replace the first summer treatment with formic acid with the advantage that the treatment can already be started early in the foraging season (preferably in mid-July)[22-23]. In sum, the treatment method shows a high effectiveness against the Varroa mite without causing contamination, but it slows down the growth of the colonies and is very time-consuming.
Varroa resistance breeding
Various traits of honeybees increase their resistance against the Varroa mite and these traits are, to a certain extent, hereditary. This includes the ability of the workers to remove the mites from each other’s bodies (grooming behaviour) as well as the ability to recognise and remove infected brood (hygienic behaviour)[24].
No varroa resistant breeding line is available in Europe to date, but varroa resistance can be taken into account by beekeepers as a breeding trait in queen breeding. To estimate the resistance, several measurements can be carried out, which differ in accuracy and the amount of time required. The easiest way is to measure the population growth of the mites over several months. However, population growth depends on numerous factors and is thus a rather inaccurate measure. A more accurate measurement of resistance would be a test of the hygienic behaviour of the colony. For this purpose, covered larvae are pierced with a needle. Subsequently, the removal of the damaged brood by the workers is measured[25]. In addition, the proportion of damaged mites on the bottom board is a good measure of grooming behaviour, but it is difficult to assess this precisely[25].
In principle, resistance breeding is a very time-consuming and only promising in the long term (decades), because the desired traits can be lost again through natural mating. Beekeepers who start resistance breeding will therefore still be dependent on varroa treatments, but will contribute to a sustainable solution for the future.
Current research
Sex pheromones
In an ongoing study at the University of Hohenheim, researchers are testing the use of sexual pheromones of the female varroa mite, to disturb the reproductive success of the mites. By spraying the combs with this pheromone, the male Varroa mites could become confused. The males could no longer distinguish between females that were already mated, not yet fertile and receptive. With this method, the reproduction rate of the mites could be reduced by 20%[26].
Book scorpions
Worldwide, several species of pseudoscorpions live naturally in beehives. The species native to Europe is the so-called book scorpion (Chelifer cancroides). The presence of the book scorpion in beehives has decreased with modern beekeeping. Chemical Varroa treatments are also deadly for the book scorpion. In addition, the scorpions need sufficient hiding places for survival in the beehive, which are often not available in modern hive systems. This decrease is regrettable as recent studies have shown that pseudoscorpions can feed on varroa mites and kill up to 9 mites per day[27-30]. However, the extent to which book scorpions can reduce a colony’s varroa infestation has not yet been investigated and their potential for varroa control is uncertain.
Fungi
Several research groups are investigating the use of entomopathogenic fungi against the varroa mite. These fungi can infect and kill arthropods (both bees and varroa mites belong to this group), but no Varroa-specific fungi are known yet. The examined fungi therefore carry the risk of infecting and harming the bees. Previous field trials have not yielded very promising results. Just a small or little effect against the varroa mite could be determined[31-32], while on the other hand some studies proved to do harm to the bees. Certain treatments resulted in increased mortality rates and a reduction in the learning ability of the bees[31,33].
Conclusion
Different treatment methods have different advantages and disadvantages. When evaluating the treatment methods, not only the effectiveness against the varroa mite should be taken into account, but also the tolerance of the bees and the risk of mites developing resistance for the method in question. Additionally, the handling for the beekeeper and possible contamination of bee products are also important factors (Table 1).
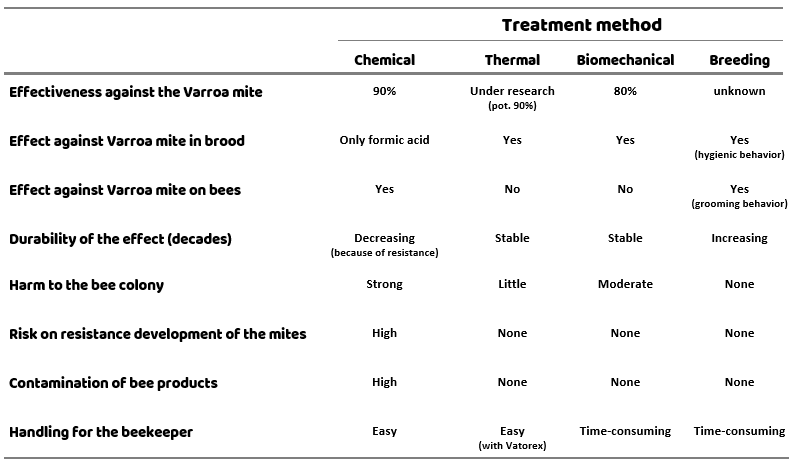
At the moment there is no treatment method available that achieves optimal results in all areas – the best solution is to combine different treatments to maximize the benefits and minimize the disadvantages. Finding a good strategy is not an easy task!
Literature
1 Fries, I., Imdorf, A. & Rosenkranz, P. Survival of mite infested (Varroa destructor) honey bee (Apis mellifera) colonies in a Nordic climate. Apidologie 37, 564-570, doi:10.1051/apido:2006031 (2006).
2 Underwood, R. M. & Currie, R. W. The effects of temperature and dose of formic acid on treatment efficacy against Varroa destructor (Acari : Varroidae), a parasite of Apis mellifera (Hymenoptera : Apidae). Experimental and Applied Acarology 29, 303-313, doi:10.1023/a:1025892906393 (2003).
3 Milani, N. The resistance of Varroa jacobsoni Oud. to acaricides. Apidologie 30, 229-234, doi:10.1051/apido:19990211 (1999).
4 Wallner, K. Varroacides and their residues in bee products. Apidologie 30, 235-248, doi:10.1051/apido:19990212 (1999).
5 Collins, A. M., Pettis, J. S., Wilbanks, R. & Feldlaufer, M. F. Performance of honey bee (Apis mellifera) queens reared in beeswax cells impregnated with coumaphos. Journal of Apicultural Research 43, 128-134 (2004).
6 Pettis, J. S., Collins, A. M., Wilbanks, R. & Feldlaufer, M. F. Effects of coumaphos on queen rearing in the honey bee, Apis mellifera. Apidologie 35, 605-610, doi:10.1051/apido:2004056 (2004).
7 Gunes, N. et al. Stress responses of honey bees to organic acid and essential oil treatments against varroa mites. Journal of Apicultural Research 56, doi:10.1080/00218839.2017.1291229 (2017).
8 Martin-Hernandez, R. et al. Short term negative effect of oxalic acid in Apis mellifera iberiensis. Spanish Journal of Agricultural Research 5, 474-480 (2007).
9 Higes, M., Meana, A., Suarez, M. & Llorente, J. Negative long-term effects on bee colonies treated with oxalic acid against Varroa jacobsoni Oud. Apidologie 30, 289-292, doi:10.1051/apido:19990404 (1999).
10 Rademacher, E. & Harz, M. Oxalic acid for the control of varroosis in honey bee colonies – a review. Apidologie 37, 98-120, doi:10.1051/apido:2005063 (2006).
11 Mert, G. & Yucel, B. Efficacy Levels of Organic Acids are Used for Controlling Varroa (Varroa jacobsoni Qudemans) and Their Effects on Colony Development of Honey Bees (Apis mellifera L.). Journal of Animal and Veterinary Advances 10, 1106-1111 (2011).
12 Ostermann, D. J. & Currie, R. W. Effect of formic acid formulations on honey bee (Hymenoptera : Apidae) colonies and influence of colony and ambient conditions on formic acid concentration in the hive. Journal of Economic Entomology 97, 1500-1508 (2004).
13 Giovenazzo, P. & Dubreuil, P. Evaluation of spring organic treatments against Varroa destructor (Acari: Varroidae) in honey bee Apis mellifera (Hymenoptera: Apidae) colonies in eastern Canada. Experimental and Applied Acarology 55, 65-76, doi:10.1007/s10493-011-9447-3 (2011).
14 Westcott, L. C. & Winston, M. L. Chemical acaricides in Apis mellifera (Hymenoptera : Apidae) colonies; do they cause nonlethal effects? Canadian Entomologist 131, 363-371 (1999).
15 Mattila, H. R., Otis, G. W., Daley, J. & Schulz, T. Trials of Apiguard, a thymol-based miticide Part 2. Non-target effects on honey bees. American Bee Journal 140, 68-70 (2000).
16 Floris, I., Satta, A., Cabras, P., Garau, V. L. & Angioni, A. Comparison between two thymol formulations in the control of Varroa destructor: Effectiveness, persistence, and residues. Journal of Economic Entomology 97, 187-191, doi:10.1603/0022-0493-97.2.187 (2004).
17 Robert Sieber, J.-D. C. Wieder etwas höhere Winterverluste. Schweizerische Bienenzeitung (2017).
18 Bogdanov, S., Charriere, J. D., Imdorf, A., Kilchenmann, V. & Fluri, P. Determination of residues in honey after treatments with formic and oxalic acid under field conditions. Apidologie 33, 399-409, doi:10.1051/apido:2002029 (2002).
19 Le Conte, Y., Arnold, G. & Desenfant, P. Influence of brood temperature and hygrometry variations on the development of the honey-bee ectorparasite Varroa jacobsoni (Mesostigmata, Varroidae). Environmental Entomology 19, 1780-1785 (1990).
20 Goras, G. et al. Hyperthermia -a non-chemical control strategy against varroa. Journal of the Hellenic Veterinary Medical Society 66, 249-256 (2015).
21 Calderone, N. W. Evaluation of drone brood removal for management of Varroa destructor (Acari : Varroidae) in colonies of Apis mellifera (Hymenoptera : Apidae) in the northeastern United States. Journal of Economic Entomology 98, 645-650 (2005).
22 Glanzmann, J. Bannwabenverfahren – eine säurefreie Varroabekämpfung. Schweizerische Bienenzeitung (2017).
23 Helen Albertin-Eicher, P. A.-E. Honig – Milben – Zuckerwasser. Schweizerische Bienenzeitung (2017).
24 Harbo, J. R. & Harris, J. W. Heritability in honey bees (Hymenoptera : Apidae) of characteristics associated with resistance to Varroa jacobsoni (Mesostigmata : Varroidae). Journal of Economic Entomology 92, 261-265 (1999).
25 Buchler, R., Berg, S. & Le Conte, Y. Breeding for resistance to Varroa destructor in Europe. Apidologie 41, 393-408, doi:10.1051/apido/2010011 (2010).
26 Ziegelmann, B. & Rosenkranz, P. Mating disruption of the honeybee mite Varroa destructor under laboratory and field conditions. Chemoecology 24, 137-144, doi:10.1007/s00049-014-0155-4 (2014).
27 Read, S., Howlett, B. G., Donovan, B. J., Nelson, W. R. & van Toor, R. F. Culturing chelifers (Pseudoscorpions) that consume Varroa mites. Journal of Applied Entomology 138, 260-266, doi:10.1111/jen.12096 (2014).
28 van Toor, R. F., Thompson, S. E., Gibson, D. M. & Smith, G. R. Ingestion of Varroa destructor by pseudoscorpions in honey bee hives confirmed by PCR analysis. Journal of Apicultural Research 54, 555-562, doi:10.1080/00218839.2016.1184845 (2015).
29 Donovan, B. J. & Paul, F. Pseudoscorpions: the forgotten beneficials inside beehives and their potential for management for control of varroa and other arthropod pests. Bee World 86, 83-87 (2005).
30 Fagan, L. L. et al. Varroa management in small bites. Journal of Applied Entomology 136, 473-475, doi:10.1111/j.1439-0418.2011.01666.x (2012).
31 Holt, M. Investigations into the suitabiity of entomopathogenic fungi and the identification of hyperparasitic fungi as antagonists of the honeybee mite Varroa destructor. Dissertation (2010).
32 James, R. R., Hayes, G. & Leland, J. E. Field trials on the microbial control of varroa with the fungus Metarhizium anisopliae. American Bee Journal 146, 968-972 (2006).
33 Kanga, L. H. B., Jones, W. A. & James, R. R. Field trials using the fungal pathogen, Metarhizium anisopliae (Deuteromycetes : Hyphomycetes) to control the Ectoparasitic mite, Varroa destructor (Acari : Varroidae) in honey bee, Apis mellifera (Hymenoptera : Apidae) colonies. Journal of Economic Entomology 96, 1091-1099, doi:10.1603/0022-0493-96.4.1091 (2003).